Which of the Following Best Describes a Single Replacement Reaction
The three general categories of single-replacement reactions are. How much heat is needed for this total process.

Single Replacement Reaction Definition And Examples
In this general reaction element A is a metal and replaces element B also a metal in the compound.

. Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. When you look at the chemical equation for a reaction a single-displacement reaction is characterized by one cation or anion trading places with another to form a new product. Its easy to spot when one of the reactants is an element and the other is a compound.
Skeletal reaction is writing an equation without following the law of conservative. One element takes the place of another in a compound B. What best describes a decomposition reaction.
Question 3 of 10 Which of the following best describes a single-replacement reaction. What type of chemical reaction is illustrated in the following example. There are two types of single replacement reactions.
Helpful 0 Not Helpful 1 Add a Comment. A compound breaks apart into separate elements. Note the arrow has this symbol Δ over it Δ Na2CO3s Na2Os CO2g Sodium carbonate is heated to give sodium oxide and carbon dioxide.
Which of the following is a single replacement reaction. Consider the following equation. ZnCuCl2 Cu ZnCl2.
Combination reaction C single-replacement reaction D double-replacement reaction E neutralization reaction. Which of the statements below best describes the following reaction. A single-replacement reaction also called a single-displacement reaction is one in which a pure element and a compound react chemically so that the products include another pure element and a different compound via exchanging of two species.
If youre seeing this message it means were having trouble loading external resources on our website. 1 See answer Advertisement Advertisement laurenkropp is waiting for your help. Zns CuSO4aq - Cu and ZnSO4.
Well its D Mg 2HCl ----- MgCl2 H2. HINT you will need to perform two calculations and then add up the results. Two elements combine to form a compound D.
Two elements switch places in. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. Sodium carbonate decomposes with heat.
A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. The positive hydrogen ion on the Chlorine has been replaced by a positive sodium ion on the Chlorine. The reaction above is classified as.
The general form of a single-replacement also called single-displacement reaction is. Simpler compounds from a complex compound. What are the products from the following single-replacement reaction.
HClaq KOHaq KClaq H2Ol. Because Magnesium Replaces Hydrogen. Which of the statements below best describes the following reaction.
Two elements switch places in two compounds. Recognizing a Single-Displacement Reaction. A halogen replaces another halogen that is in solution.
Ice with a mass of 15 grams is melted AND then the temperature of the now liquid water is raised from 0 degrees Celsius up to 95 degree Celsius. ABC B AC. In other words it is where an element reacts with a compound and replaces one component of the compound.
While the Sodium ion on. A compound breaks apart into separate elements C. Balance polyatomic ions as a single unit check each reactant and product to verify the coefficients.
Which of the statements below best describes the following reaction. See answer 1 Best Answer. Add your answer and earn points.
All of the above. A single substance breaks down into. People also asked Which of the following describes a single-replacement reaction.
This is a double replacement reaction that is also a neutralization It is a double replacement because the reaction starts with two compounds and ends with two compounds where the positive and negative ions have changed places. Two elements combine to form a compound. One element takes the place of another in a compound.
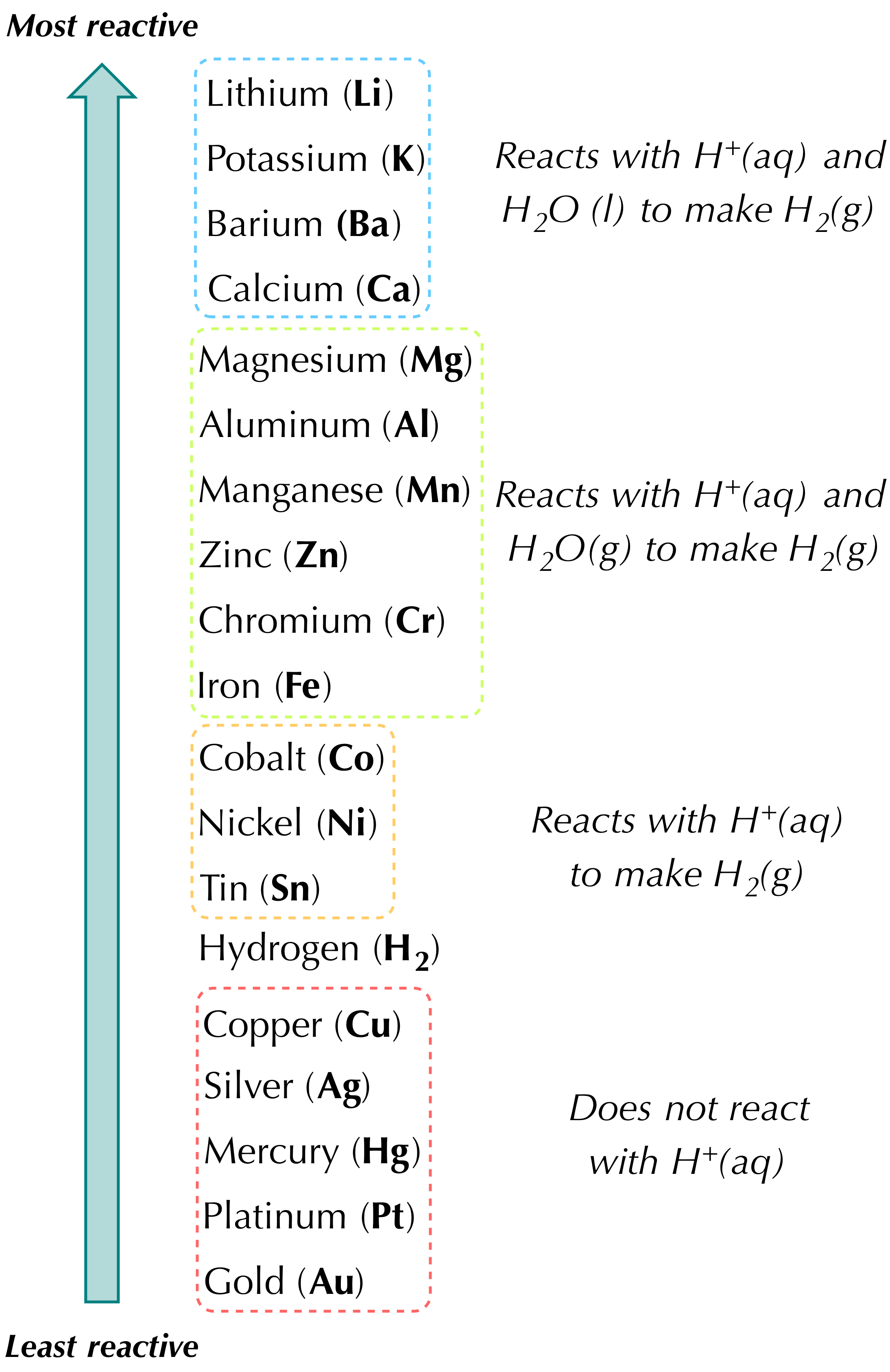
Which of the following best describes a single-replacement reaction. Definition of single replacement or single displacement reactions. Predicting and determining the products using the reactivity series.
Mathrm Zn s2 mathrm HCl a q rightarrow mathrm ZnCl_ 2 a qmathrm H_ 2 g The reaction can be made to occur more slowly by 1 raising the temperature and using a single piece of zinc rather than powdered zinc of the same mass 2 lowering the. A metal replaces another metal that is in solution. 1172 A BC AC B.
A single replacement reaction occur when an element in a substance is replaced by other element. Mg 2HCl MgCl2 H2.
Single Replacement Reactions Article Khan Academy
What Are The Differences Between Single And Double Displacement Reactions Quora

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube
Belum ada Komentar untuk "Which of the Following Best Describes a Single Replacement Reaction"
Posting Komentar